Abstract
Background
Longer time to achieve complete remission (CR) and recover counts after initial induction chemotherapy in acute myeloid leukemia (AML) patients is associated with decreased overall survival (OS) and relapse-free survival. Prolonged time to count recovery is associated with increased risk of infection and bleeding, and can result in delay of planned subsequent therapies including further chemotherapy or allogeneic transplant. While CR with count recovery after induction chemotherapy typically occurs within 4-5 weeks, there is a paucity of data for count recovery after post-remission (also known as consolidation) chemotherapy. We sought to investigate the time to neutrophil and platelet recovery after the first cycle of consolidation chemotherapy and thereby to identify optimal post-remission chemotherapy regimens.
Methods
We retrospectively identified adults over 18 years with newly diagnosed WHO-defined AML/high grade myeloid neoplasms (>10% blasts) who received high intensity induction chemotherapy (cladribine, cytarabine, mitoxantrone, and G-CSF, also known as CLAG-M or GCLAM) between 5/1/2014 and 12/31/2019. Patients with variations to the CLAG-M backbone such as sorafenib, midostaurin, gemtuzumab ozogamicin, and decitabine were included as well. This study was approved by the local Institutional Review Board. Consolidation treatment was defined as the first cycle of therapy given once patients achieved CR in marrow by standard criteria and divided into 3 main types for analysis: CLAG-M, CLAG-M without mitoxantrone (CLAG or GCLA) and high dose cytarabine (HiDAC). Patients who did not receive post-remission therapy with these regimens (n=23) or had refractory disease/died before consolidation therapy (n=180) were excluded from analysis. Many patients were enrolled on a single-center clinical trial of CLAG-M with dose-escalated mitoxantrone (NCT02044796). The primary endpoint was evaluation of the number of days from the start of consolidation chemotherapy to the day when absolute neutrophil count (ANC) > 1,000/ml (defined as neutrophil recovery) and platelet count > 100,000/ml (defined as platelet recovery). Early death was defined as death within 28 days of receiving consolidation therapy.
Results
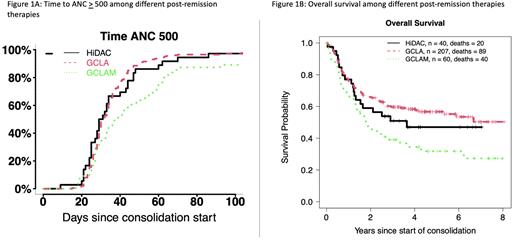
We identified 307 patients who met our inclusion criteria; of these, 207 patients (67%) received CLAG therapy, 60 patients (20%) with CLAG-M and 40 patients (13%) with HiDAC. Compared to HiDAC, the median age of patients who received CLAG or CLAG-M was lower (p=0.013). The majority of patients were treated off study in all groups. The highest proportion of patients treated on study received CLAG therapy (36%, p=0.03). Early death was a rare event overall (1% for CLAG; 2% for CLAG-M and HiDAC), and no significant differences in ELN 2017 risk, performance status, baseline WBC, platelets, creatinine, albumin, or TRM score were identified among the three groups. Median time to neutrophil recovery was 36 days for HiDAC, 37 days for CLAG, and 50.5 days for CLAG-M (Figure 1A). Median time to platelet recovery was 48 days for HiDAC, 52 days for CLAG, and 94 days for CLAG-M.
In multivariable cause-specific hazard regression models controlling for age and study enrollment, no differences were noted between CLAG and HiDAC in time to neutrophil recovery (hazard ratio [HR] 1.2, 95% confidence interval [CI] 0.81-1.76), platelet recovery (HR 1.08, 95% CI 0.71-1.64), or both neutrophil and platelet recovery together (HR 1.06, 95% CI 0.69-1.61). When compared to HiDAC, CLAG-M was associated with a trend towards longer time to neutrophil recovery (HR 0.69, 95% CI 0.43-1.09), platelet recovery (HR 0.73, 95% CI 0.44-1.21), and both neutrophil and platelet recovery together (HR 0.68, 95% CI 0.41-1.13).
Controlling for age and study enrollment status, CLAG-M was associated with significantly worse 8-year OS compared to HiDAC (p=0.030), but no significant difference was identified between CLAG and HiDAC (p=0.99) (Figure 1B).
Conclusions
Post-remission therapy with CLAG is another viable alternative to HiDAC with no significant differences in neutrophil recovery, platelet recovery, and OS. Early death was uncommon for all three groups, but a post-remission course of CLAG-M was associated with decreased OS. Future work will examine the effect of particular cytogenetic and molecular markers on count recovery after post-remission courses.
Disclosures
Othus:Merck: Consultancy; Glycomimetics: Consultancy; Biosight: Consultancy; Daiichi Sankyo: Consultancy; Celgene: Consultancy. Halpern:Incyte Pharmaceuticals: Research Funding; Jazz Pharmaceuticals: Research Funding; Gilead Sciences: Research Funding; Imago Biosciences: Research Funding; Novartis: Research Funding; Tolero Pharmaceuticals: Research Funding; Bayer Pharmaceuticals: Research Funding; Abbvie: Consultancy; Karyopharm Therapeutics: Research Funding; Notable Labs: Consultancy. Walter:BioLineRx, LTd: Consultancy, Research Funding; Celgene, Inc: Consultancy, Research Funding; Genentech: Consultancy; GSK: Consultancy; ImmunoGen: Research Funding; Janssen Global Services, LLC: Consultancy; Janssen Research and Development: Research Funding; Agios: Consultancy, Research Funding; Amphivena Therapeutics, Inc: Current equity holder in publicly-traded company; Amgen: Consultancy, Research Funding; Pfizer, Inc: Consultancy, Research Funding; MacroGenics: Consultancy, Research Funding; New Link Genetics: Consultancy; Jazz Pharmaceuticals: Consultancy, Research Funding; Kite Pharma, Inc: Consultancy; Kronos Bio, Inc: Consultancy; Kura Oncology: Consultancy, Research Funding; Race Oncology LTD: Consultancy; Selvita: Research Funding; Stemline Therapeutics: Research Funding; Aptevo Therapeutics: Consultancy, Research Funding; Arog Pharmaceuticals: Research Funding; Astellas Pharma US, Inc: Consultancy; Bristol Myers Squibb, Inc: Consultancy; Boston Biomedical, Inc: Consultancy; AbbVie: Consultancy; BerGenBio, ASA: Consultancy; Orum Therapeutics, Inc.: Consultancy. Percival:Pfizer: Research Funding; Trillium: Research Funding; Oscotec: Research Funding; Cardiff Oncology: Research Funding; Glycomimetics: Research Funding; Biosight: Research Funding; Celgene/BMS: Research Funding; Abbvie: Research Funding; Ascentage: Research Funding; Telios: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal